Genedrive® MT-RNR1 ID Kit
The world’s first point of care genetic test used to influence neonatal management in an acute setting and reduce aminoglycoside induced hearing loss (AIHL)

- A rapid genetic test designed to be used in time critical situations, prior to antibiotic treatment prescription
- Reduces the risk of antibiotic induced hearing loss by detecting a patients MT-RNR1 gene variant status
- A non-invasive test, using buccal swabs to collect samples
- Simple to use test kits, with sealed, single use cartridges
- Easy adoption into existing neonatal admissions process without disrupting normal standard of care
With a simple to follow test procedure:
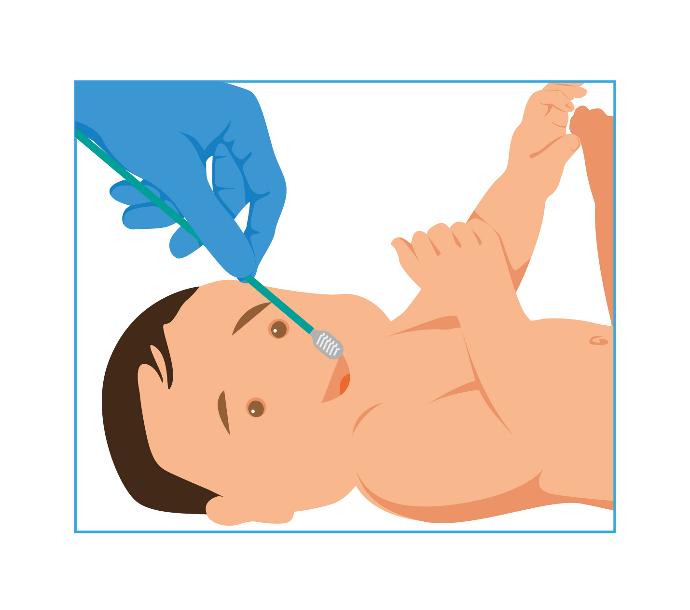
1- Swab
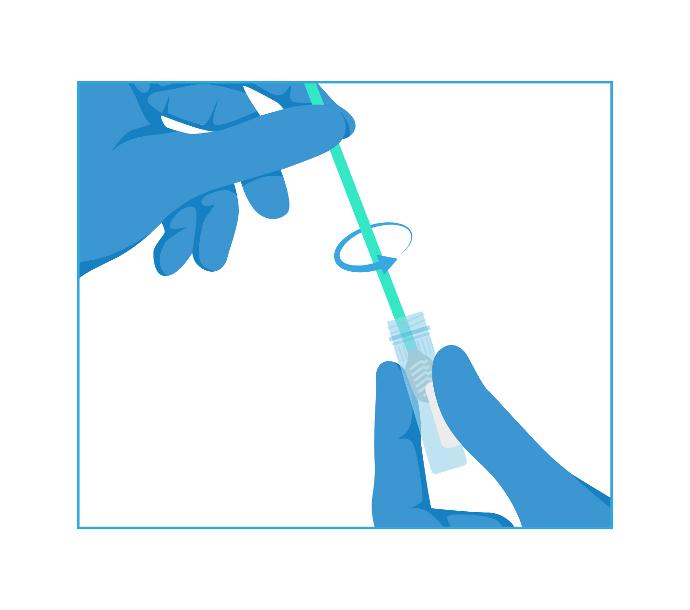
2- Mix
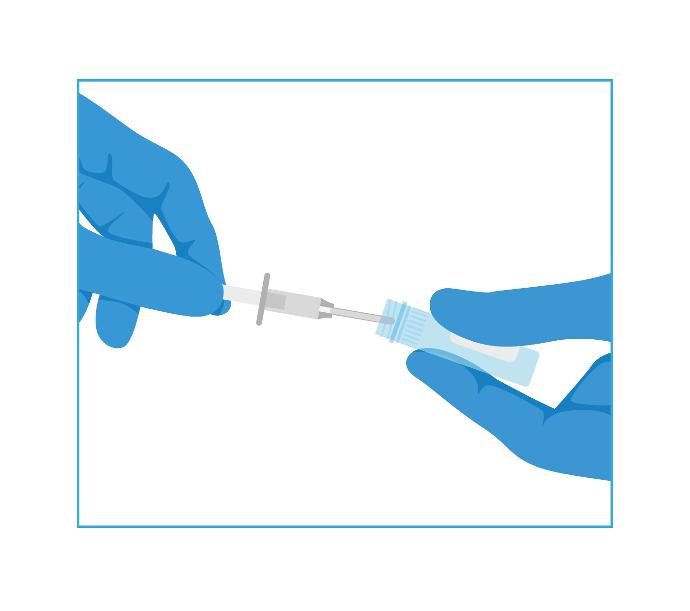
3- Transfer
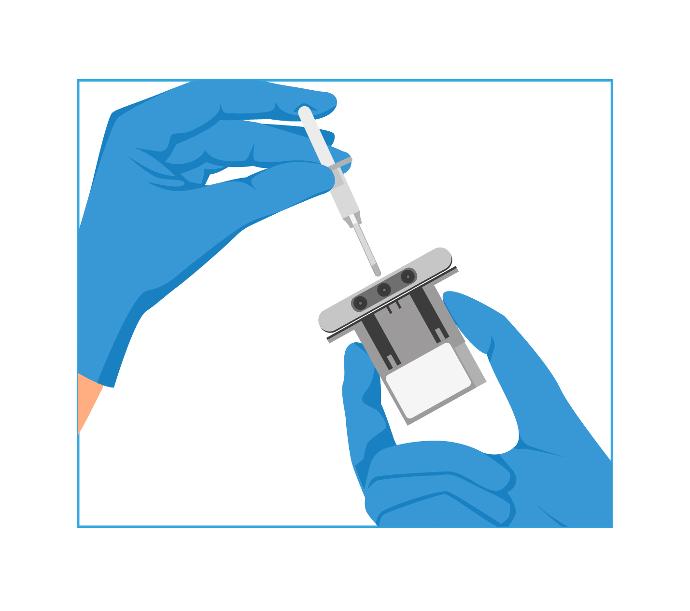
4- Reconstitute Assay
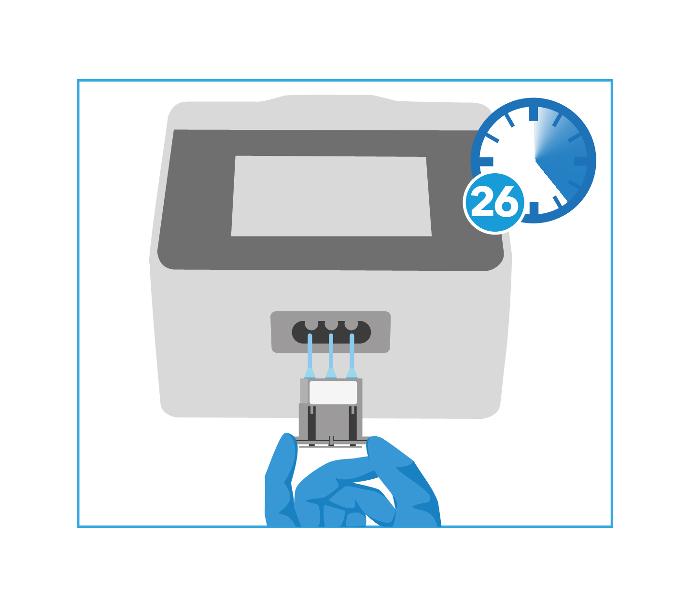
5- Run Test
The Genedrive® MT-RNR1 ID Kit is used on the Genedrive® System to provide an automated result of an individual's MT-RNR1 m.1555A>G variant status to inform the clinician ahead of antibiotic treatment decisions.
![]()
Individuals with the MT-RNR1 m.1555A>G gene variant develop profound irreversible hearing loss if exposed to aminoglycosides (such as gentamicin). Population based studies estimate a prevalence of 1:500 (0.2%).
Implementing the Genedrive® MT-RNR1 ID Kit into routine clinical practice allows clinicians to prescribe an alternative antibiotic. For example, cephalosporin antibiotics might be prescribed (alternatively to aminoglycoside antibiotics) to those patients who have tested positive for the MT-RNR1 m.1555A>G variant, using the Genedrive® System. Clinicians can therefore reduce the risk of antibiotic induced hearing loss (AIHL).
This enables patients to get the right treatment at the right time, tailored to their results.
![]()

![]()

With a time to result of 26 minutes, the Genedrive® MT-RNR1 ID Kit allows clinicians to make informed treatment decisions within the golden hour, without disrupting normal standard of care.